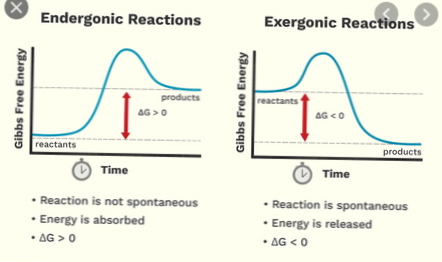
In summary, whereas, an exergonic reaction means that a reaction is spontaneous, an exothermic reaction has nothing to do with spontaneity, but that an energy is released to the surrounding.
- What is the difference between endothermic and endergonic reaction?
- Is Endergonic exothermic or endothermic?
- Is Endergonic endothermic?
- Are the terms exothermic and exergonic synonymous?
- Is sweating Exergonic or Endergonic?
- Is cooking an egg endothermic or exothermic?
- Is ice melting endothermic or exothermic?
- What is the difference between exothermic and endothermic?
- Is exothermic spontaneous?
- Which process is Endergonic?
- Why Gibbs free energy is negative?
- Is catabolism exothermic or endothermic?
What is the difference between endothermic and endergonic reaction?
They both mean that heat is absorbed, but the difference is that endothermic is a relative change in enthalpy, whilst endergonic refers to the relative change in free energy of a system. ... In an endergonic reaction, energy is absorbed from the surroundings.
Is Endergonic exothermic or endothermic?
Re: Exothermic vs Exergonic and Endothermic vs Endergonic
Exo/Endothermic represents the relative change in heat/enthalpy in a system, whereas Exer/Endergonic refers to the relative change in the free energy of a system.
Is Endergonic endothermic?
Endergonic reactions are not spontaneous. Examples of endergonic reactions include endothermic reactions, such as photosynthesis and the melting of ice into liquid water. If the temperature of the surroundings decreases, the reaction is endothermic.
Are the terms exothermic and exergonic synonymous?
Exothermic and exergonic are not synonymous. Further Explanation: Both the process releases energy in the environment although, both the term are not synonymous with one another. A reaction that is considered as exergonic reaction involves those chemical bonds of the substances that are focused on.
Is sweating Exergonic or Endergonic?
When you sweat, the system – your body – cools down as perspiration evaporates from the skin and heat flows to the surrounding area. This means sweating is an exothermic reaction.
Is cooking an egg endothermic or exothermic?
Cooking an egg is an endothermic process because added energy makes it cooked. An egg without heats stays an (uncooked) egg. In this reaction, energy is absorbed.
Is ice melting endothermic or exothermic?
As a result, the temperature of the ice rises and it turns into water! Basically, melting ice is an endothermic reaction because the ice absorbs (heat) energy, which causes a change to occur.
What is the difference between exothermic and endothermic?
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.”
Is exothermic spontaneous?
A roaring bonfire is an example of a spontaneous reaction, since it is exothermic (there is a decrease in the energy of the system as energy is released to the surroundings as heat). ... The combination of energy decrease and entropy increase dictates that combustion reactions are spontaneous reactions.
Which process is Endergonic?
Exergonic reactions involve the breaking of bonds; endergonic reactions involve the formation of bonds. b. Exergonic reactions release energy; endergonic reactions absorb it. ... In exergonic reactions, the reactants have less chemical energy than the products; in endergonic reactions, the opposite is true.
Why Gibbs free energy is negative?
Endergonic and exergonic reactions
A negative ∆G means that the reactants, or initial state, have more free energy than the products, or final state. Exergonic reactions are also called spontaneous reactions, because they can occur without the addition of energy.
Is catabolism exothermic or endothermic?
With catabolism, the heat of formation of the products ( like CO2 ) is very low, and the exothermic heat energy that is produced drives all the other endothermic reactions required for life. This is why the entire catabolism sequence of reactions is exothermic.
 Differbetween
Differbetween